COVID Vaccines
Unlock the Pulse of the Present
Subscribe Now for Real-time Updates on the Latest Stories!
Latest Articles
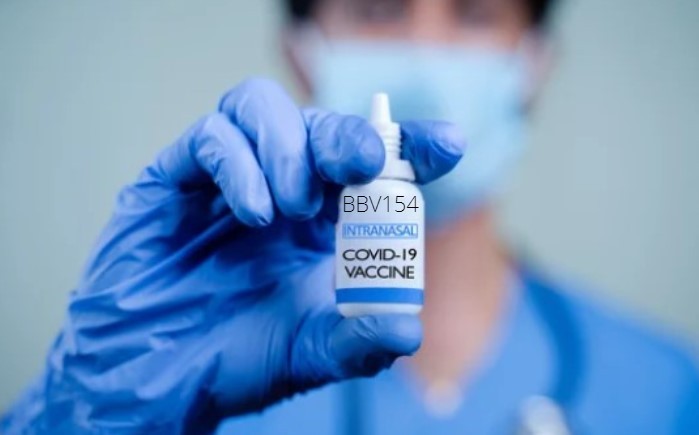
Intranasal Covid vaccine BBV154 is safe, regulators approvals awaited: Bharat Biotech
By
TheNewsFacts
Japan Approves COVAXIN booster dose for Travelers; reinforces the effectiveness of Universal Covid Vaccine
By
TheNewsFacts