COVAXIN India’s indigenously developed and manufactured COVID-19 Vaccine continues to demonstrate its scientific commitment by sharing and publishing data generation and data transparency. So far, Bharat Biotech has published full data of all research studies of its COVAXIN®, India’s first indigenous COVID-19 vaccine in 10 globally recognized scientific journals.
In a timely approach to peer review, the company has already published as many as Ten research studies on the safety and efficacy of COVAXIN® in five globally reputed peer-reviewed journals in a span of just twelve months.
COVAXIN®, a whole-virion inactivated coronavirus vaccine, has many firsts to its credit in data transparency. It is the first and only product to have published any data from human clinical trials in India. It is the only product to have any data on emerging variants. It is also the first and only COVID-19 vaccine to have efficacy data in Indian populations.
COVAXIN data published in 10 leading scientific publications – a first amongst peers
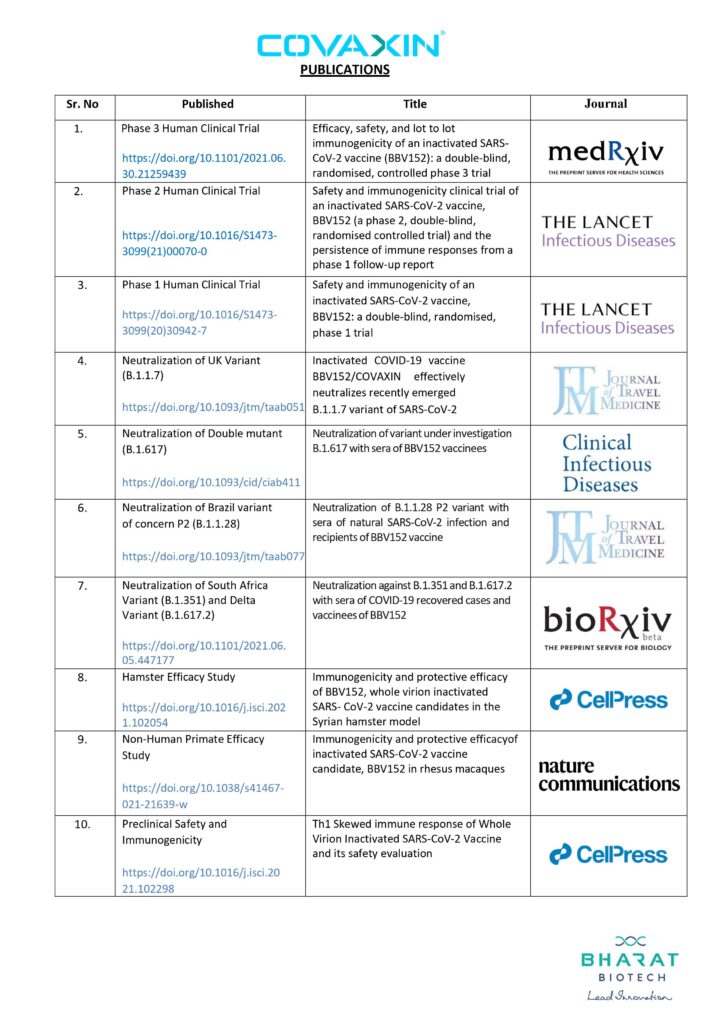
In vaccine development, preclinical studies involve the testing of vaccine candidates in laboratory animals. Bharat Biotech completed three preclinical studies, which are published in Cellpress, a peer-reviewed journal. The studies on COVAXIN’s Phase I (done to assess a vaccine’s safety, immune response and to determine the right dosage), and Phase II clinical trial (carried out to assess the safety and the ability of the vaccine to generate an immune response) are published by the peer-reviewed journal, The Lancet – Infectious Diseases.
The full data from studies on COVAXIN’s neutralization of variants are already published at the peer-reviewed journals of bioRxiv, Clinical Infectious Diseases, and Journal of Travel Medicine.
The study on the neutralization of Beta and Delta variants (B.1.351 and B.1.617.2 respectively) is published at bioRxiv, and the study on B1.1.28 variant, at Journal of Travel Medicine, while the studies on B.1.617 variant and Alpha variant (B.1.1.7) are published at Clinical Infectious Disease, and Journal of Travel Medicine respectively.
The published studies are widely cited for the rigor and breadth that Bharat Biotech brings to its clinical trials. Currently, data from both efficacy and safety follow-up of COVAXIN’s Phase III trial is being analyzed and compiled. Upholding its uncompromising commitment to integrity, the company will make Phase III trials data from the final analysis public in July 2021.
Today, COVAXIN also received Hungarian GMP certification for its manufacturing practices.


